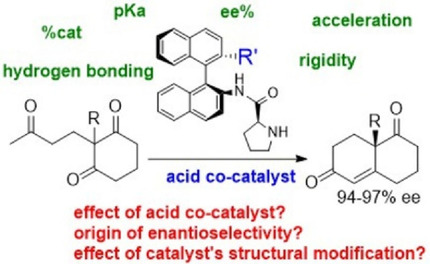
The organocatalyzed synthesis of the Wieland‐Miescher ketone (WMK) via N‐sulfonyl‐binamprolinamide catalysis was investigated using experimental and computational tools. A mechanistic proposal is presented describing the origin of the high enantioselectivity, which rivals that of aldolases. The computational study reveals that the role of the prolinamide catalyst is to lower the reaction barrier and determine the stereoselectivity of the product achieved, while the role of the carboxylic acid is to facilitate proton transfer steps. The effect of the acid co‐catalyst was confirmed by experiments. The role of the structure of the BINAM backbone and the effect of the sulfonamide group are uncovered experimentally and computationally. Calculations show that a rigid highly defined catalytic pocket due to covalent and steric interactions induces conformational changes in the triketone substrate to maximize interactions.