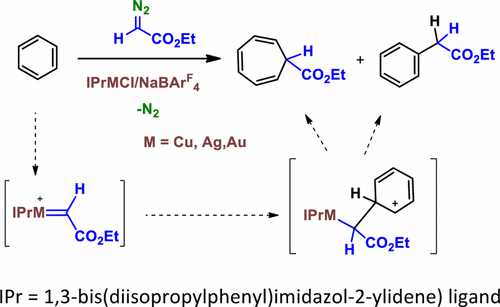
The catalytic functionalization of the Csp2–H bond of benzene by means of the insertion of the CHCO2Et group from ethyl diazoacetate (N2═CHCO2Et) has been studied with the series of coinage-metal complexes IPrMCl (IPr = 1,3-bis(diisopropylphenyl)imidazol-2-ylidene) and NaBArF4 (BArF4 = tetrakis(3,5-bis(trifluoromethyl)phenyl)borate). For Cu and Ag, these examples constitute the first use of such metals toward this transformation, which also provides ethyl cyclohepta-2,4,6-trienecarboxylate as a byproduct from the so-called Buchner reaction. In the case of methyl-substituted benzenes, the reaction exclusively proceeds onto the aromatic ring, the Csp3–H bond remaining unreacted. A significant coinage-metal effect has been observed, since the gold catalyst favors the formation of the insertion product into the Csp2–H bond whereas copper and silver preferentially induce the formation of the cycloheptatriene derivative. Experimental studies and theoretical calculations have explained the observed selectivity in terms of the formation of a common Wheland intermediate, resembling an electrophilic aromatic substitution, from which the reaction pathway evolves into two separate routes to each product.