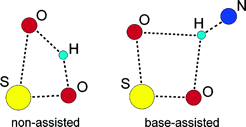
A computational study with density functional theory (DFT) is carried out on the reaction between methyl sulfinyl chloride (MSC) and methanol in the presence of trimethylamine, a process which is a general model for two different methods used in practice for the obtention of chiral sulfoxides through dynamic kinetic resolution. Two mechanistic options are considered: in one of them, chloride is initially displaced by the base (ion pair mechanism), whereas in the other, chloride stays bound to sulfur until its final displacement by methoxy (neutral mechanism). In both cases, the approach of the alcohol to sulfur is coupled with a hydrogen transfer from methanol to the oxo group of MSC in a single concerted transition state. The presence of a trimethylamine molecule facilitates substantially the reaction by reducing the nucleophilic substitution barrier by more than 10 kcal/mol through the formation of a N-H bond with the hydrogen atom being transferred. The neutral mechanism presents a free energy barrier lower than the ion pair alternative and is thus preferred.