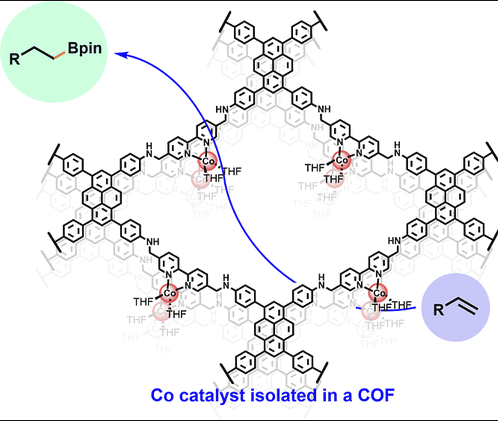
Site isolation of unstable catalytic species within a reticular material is an appealing strategy for mitigating catalyst decomposition while creating catalysts with improved activity and robustness. The covalent organic framework (COF) based on single cobalt sites coordinated to 2,2′-bipyridine (COFbpyCo) exploits the isolated nature of the cobalt sites to stabilize low-valent single sites and catalyze the hydroboration of olefins. Terminal alkyl boronate esters were obtained using 1 mol % COFbpyCo loading starting from terminal alkenes and internal alkenes, thanks to a tandem isomerization-hydroboration process. The catalytic activity of COFbpyCo was maintained after being recycled six times. Experimental and computational studies suggested the {(bpy•–)CoI(THF)2} moiety as the active catalytic species within the COF. The mechanism follows an oxidative boryl migration, followed by isomerization and reductive elimination to form the boronate ester.