Graphical Abstract
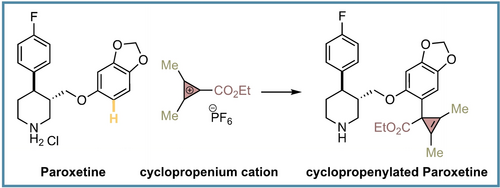
A new late-stage functionalization of complex molecules enables the installation of cyclopropene rings with excellent efficiency, thus reaching an uncharted cyclopropenylated chemical space. The cyclopropene ring generally improves the metabolic stability of the cyclopropenylated drug molecules and serves as a unique scaffold to access broad range of medicinally-relevant motifs.
Abstract
Herein, we disclose the first regio-, site- and chemoselective late-stage (hetero)aryl C−H bond cyclopropenylation with cyclopropenium cations (CPCs). The process is fast, operationally simple and shows an excellent functional group tolerance in densely-functionalized drug molecules, natural products, agrochemicals and fluorescent dyes. Moreover, we discovered that the installation of the cyclopropene ring in drug molecules could not only be used to shield against metabolic instability but also as a synthetic tool to reach medicinally-relevant sp3-rich scaffolds exploiting the highly-strained nature of the cyclopropene ring with known transformations.