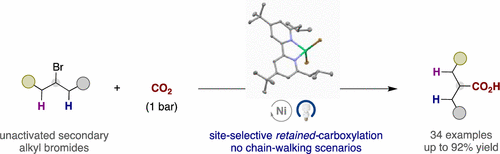
Herein, we report the direct carboxylation of unactivated secondary alkyl bromides enabled by the merger of photoredox and nickel catalysis, a previously inaccessible endeavor in the carboxylation arena. Site-selectivity is dictated by a kinetically controlled insertion of CO2 at the initial C(sp3)–Br site by the rapid formation of Ni(I)–alkyl species, thus avoiding undesired β-hydride elimination and chain-walking processes. Preliminary mechanistic experiments reveal the subtleties of stereoelectronic effects for guiding the reactivity and site-selectivity.