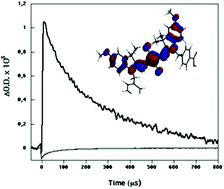
The photovoltaic performance of liquid electrolyte and solid-state dye sensitized solar cells, employing a squarilium methoxy cyanide dye, are evaluated in terms of interfacial electron transfer kinetics. Dye adsorption to the metal oxide film resulted in a mixed population of aggregated and monomeric sensitizer dyes. Emission quenching data, coupled with transient absorption studies, indicate that efficient electron injection was only achieved by the monomeric dyes, with the aggregated dye population having an injection yield an order of magnitude lower. In liquid electrolyte devices, transient absorption studies indicate that photocurrent generation is further limited by slow kinetics of the regeneration of monomeric dye cations by the iodide/iodine redox couple. The regeneration dynamics are observed to be too slow (>> 100 µs) to compete effectively with the recombination of injected electrons with dye cations. In contrast, for solid-state devices employing the organic hole conductor spiro-OMeTAD, the regeneration dynamics are fast enough (<< 1 µs) to compete effectively with this recombination reaction, resulting in enhanced photocurrent generation.