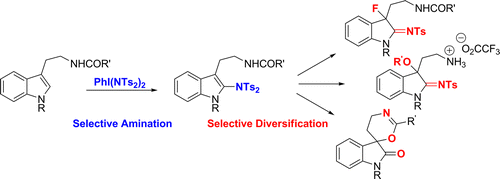
Defined hypervalent iodine reagents of the general structure PhI[N(SO2R)(SO2R′)]2 promote the selective direct C–H-amination of the indole core of various tryptamines. Starting from a general C2-amination strategy, subsequent transformations enable a variety of site-selective functionalizations, which proceed with noteworthy high chemoselectivity and provide an overall access to structurally diversified products.