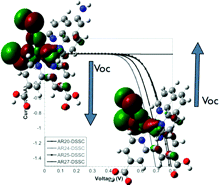
A series of heteroleptic ruthenium(II) polypyridyl complexes containing phenanthroline ligands have been designed, synthesized, and characterized. The spectroscopic and electrochemical properties of the complexes have been studied in solution and adsorbed onto semiconductor nanocrystalline metal oxide particles. The results show that for two of the ruthenium complexes, bearing electron-donating (-NH2) or electron-withdrawing (-NO2) groups, the presence of the redox-active I–/I3– electrolyte produces important changes in the interfacial charge transfer processes that limit the device performance. For example, those dyes enhanced the electron recombination reaction between the photoinjected electrons at TiO2 and the oxidized redox electrolyte. In an effort to understand the details of such striking observations, we have monitored the charge transfer reactions taking place at the different interfaces of the devices using time-resolved single photon counting, laser transient spectroscopy, and light-induced photovoltage measurements.