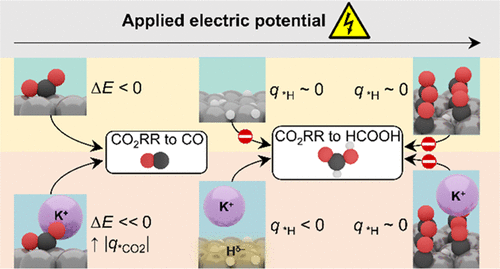
Understanding the role of cations in the electro-chemical CO2 reduction (CO2RR) process is of fundamental importance for practical application. In this work, we investigate how cations influence HCOOH and CO formation on PdMLPt-(111) in pH 3 electrolytes. While only (a small amount of adsorbed) CO forms on PdMLPt(111) in the absence of metal cations, the onset potential of HCOOH and CO decreases with increasing cation concentrations. The cation effect is stronger on HCOOH formation than that on CO formation on PdMLPt(111). Density functional theory simulations indicate that cations facilitate both hydride formation and CO2 activation by polarizing the electronic density at the surface and stabilizing *CO2-. Although the upshift of the metal work function caused by high coverage of adsorbates limits hydride formation, the cation-induced electric field counterbalances this effect in the case of *H species, sustaining HCOOH production at mild negative potentials. Instead, at the high *CO coverages observed at very negative potentials, surface hydrides do not form, preventing the HCOOH route both in the absence and presence of cations. Our results open the way for a consistent evaluation of cationic electrolyte effects on both activity and selectivity in CO2RR on Pd-Pt catalysts.