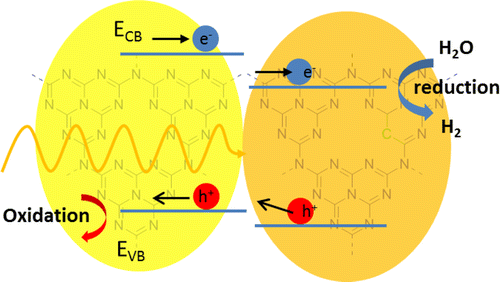
Herein, we report the facile synthesis of an efficient roll-like carbon nitride (C3N4) photocatalyst for hydrogen production using a supramolecular complex composed of cyanuric acid, melamine, and barbituric acid as the starting monomers. Optical and photocatalytic investigations show, along with the known red shift of absorption into the visible region, that the insertion of barbituric acid results in the in situ formation of in-plane heterojuctions, which enhance the charge separation process under illumination. Moreover, platinum as the standard cocatalyst in photocatalysis could be successfully replaced with first row transition metal salts and complexes under retention of 50% of the catalytic activity. Their mode of deposition and interaction with the semiconductor was studied in detail. Utilization of the supramolecular approach opens new opportunities to manipulate the charge transfer process within carbon nitride with respect to the design of a more efficient carbon nitride photocatalyst with controlled morphology and optical properties.