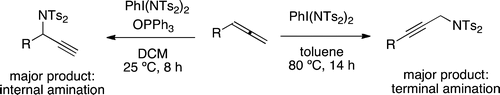
An oxidative amination of allenes using a single hypervalent iodine reagent is reported. The reaction proceeds very efficiently for monosubstituted allenes and leads to formation of the corresponding propargylic amines, either as the internal or as the terminal amine. The respective reaction outcome could be influenced in favor of the former product by addition of triphenylphosphine oxide to the iodine(III) reagent.