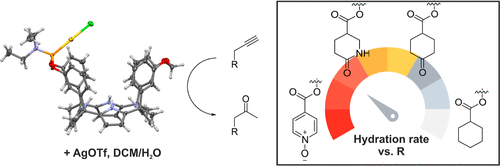
We report the synthesis and characterization of two diastereomeric phosphoramidite calix[4]pyrrole cavitands and their corresponding gold(I) complexes, 2in•Au(I)•Cl and 2out•Au(I)•Cl, featuring the metal center directed inward and outward with respect to their aromatic cavity. We studied the catalytic activity of the complexes in the hydration of a series of propargyl esters as the benchmarking reaction. All substrates were equipped with a six-membered ring substituent either lacking or including a polar group featuring different hydrogen bond acceptor (HBA) capabilities. We designed the substrates with the polar group to form 1:1 inclusion complexes of different stabilities with the catalysts. In the case of 2in•Au(I)•OTf, the 1:1 complex placed the alkynyl group of the bound substrate close to the metal center. We compared the obtained results with those of a model phosphoramidite gold(I) complex lacking a calix[4]pyrrole cavity. We found that for all catalysts, the presence of an increasingly polar HBA group in the substrate provoked a decrease in the hydration rate constants. We attributed this result to the competing coordination of the HBA group of the substrate for the Au(I) metal center of the catalysts.