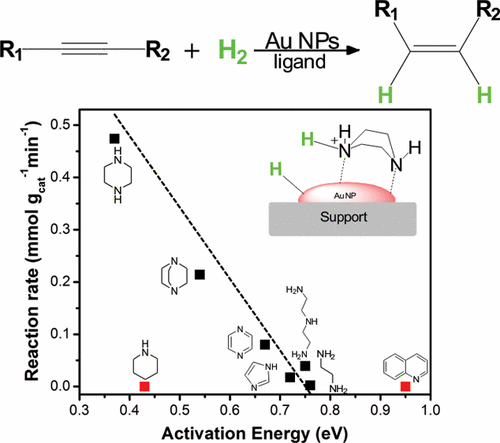
The selective hydrogenation of alkynes to alkenes is an important synthetic process in the chemical industry. It is commonly accomplished using palladium catalysts that contain surface modifiers, such as lead or silver. Here we report that the adsorption of nitrogen-containing bases on gold nanoparticles results in a frustrated Lewis pair interface that activates H2 heterolytically allowing an unexpected high hydrogenation activity. The so-formed tight-ion pair can be selectively transferred to an alkyne leading to a cis isomer, this behavior is controlled by electrostatic interactions. Activity correlates with H2 dissociation energy, which depends on the basicity of the ligand and its reorganization when activating hydrogen. High surface occupation and strong Au atom-ligand interactions might affect the accessibility and stability of the active site making the activity prediction a multiparameter function. The remarkable promotional effect found for nitrogen-containing bases with two heteroatoms was mechanistically described as a strategy to boost gold activity.