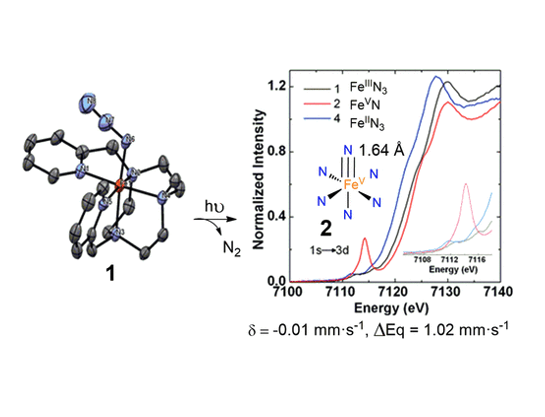
Iron complex [FeIII(N3)(MePy2tacn)](PF6)2 (1), containing a neutral triazacyclononane-based pentadentate ligand, and a terminally bound azide ligand has been prepared and spectroscopically and structurally characterized. Structural details, magnetic susceptibility data and Mössbauer spectra demonstrate that 1 has a low-spin state (S = 1/2) ferric center. X-Ray diffraction analysis of 1 reveals remarkably short Fe – N (1.859 Å) and long FeN – N2 (1.246 Å) distances, respectively, and the FT-IR spectra showed an unusually low N – N stretching frequency (2019 cm-1), suggesting that the FeN – N2 bond is particularly weak. Photolysis of 1 at 470 nm or 530 nm in frozen solutions caused N2 elimination and generation of a nitride species that on the basis of Mössbauer, magnetic susceptibility, EPR, and X – ray absorption (XANES) data in conjunction with DFT computational analyses is formulated as [FeV(N)(MePy2tacn)]2+ (2). The results showed that 2 is a low-spin S =1/2 iron(V) species and exhibits a short Fe-N distance (1.64 Å), as deduced from EXAFS analysis. Compound 2 is only stable at cryogenic (liquid N2) temperatures, and frozen solutions as well as solid samples decompose rapidly upon warming, producing N2. The high-valent compound could also be generated in the gas phase and its reactivity against olefins, sulphides and substrates with weak C-H bonds has been studied. It proved to be a powerful two-electron oxidant that can add the nitride ligand to olefin and sulphide sites, and oxidizes cyclohexadiene substrates to benzene, in a formal H2 transfer process. In summary compound 2 constitutes the first case of an octahedral FeV(N) species prepared within a neutral ligand framework, and adds to the few examples of FeV species that could be spectroscopically and chemically characterized.