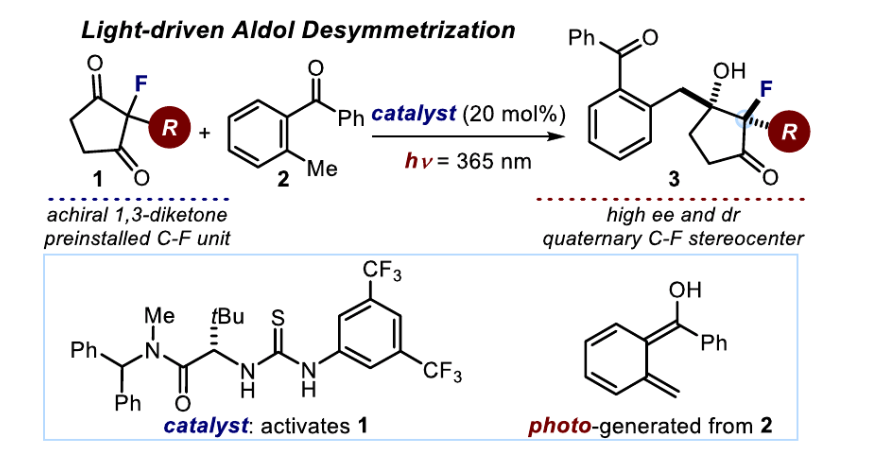
Reported herein is a light-triggered organocatalytic strategy for the desymmetrization of achiral 2-fluoro substituted cyclopentane-1,3-diketones. The chemistry is based on an intermolecular aldol reaction of photochemically generated hydroxy-o-quinodimethanes that simultaneously forges two adjacent fully substituted carbon stereocenters, one bearing a carbon-fluorine stereogenic unit. The method uses readily available substrates, a simple chiral organocatalyst, and mild reaction conditions to afford an array of highly functionalized chiral 2-fluoro-3-hydroxycyclopentanones.