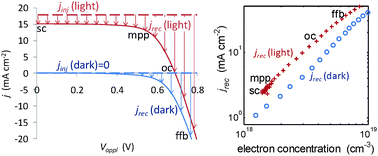
A simple and powerful approach for assessing the recombination losses in dye sensitised solarcells (DSSCs) across the current voltage curve (j–V) as a function of TiO2 electron concentration (n) is demonstrated. The total flux of electrons recombining with iodine species in the electrolyte and oxidised dye molecules can be thought of as a recombination current density, defined as jrec= jinj – j where jinj is the current of electrons injected from optically excited dye states and j is the current density collected at cell voltage (V). The electron concentration at any given operating conditions is determined by charge extraction. This allows comparison of factors influencing electron recombination rates at matched n. We show that jrec is typically 2-3 times higher under 1 sun equivalent illumination (jinj > 0) relative to dark (jinj = 0) conditions. This difference was increased by increasing light intensity, electrolyte iodine concentration and electrolyte solventviscosity. The difference was reduced by increasing the electrolyte iodide concentration and increasing the temperature. These results allowed us to verify a numerical model of complete operational cells (Barnes et al., Phys. Chem. Chem. Phys., DOI: 10.1039/c0cp01554g) and to relate the differences in jrec to physical processes in the devices. The difference between jrec in the light and dark can be explained by two factors: (1) an increase in the concentration of electron acceptor species (I3– and/or I2) when current is flowing under illumination relative to dark conditions where the current is flowing in the opposite direction, and (2) a non-trivial contribution from electron recombination to oxidised dye molecules under light conditions. More generally, the technique helps to assign the observed relationship between the components, processing and performance of DSSCs to more fundamental physical processes.