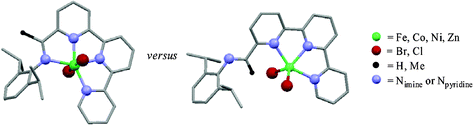
The bulky arylimino-terpyridine ligands, 6-{(2,6-i-Pr2C6H3)N=CR}-2,2:6,2-C15H10N3 [R = H (L1), Me (L2)], have been prepared in high yield from the condensation reaction of the corresponding carbonyl compound with one equivalent of 2,6-diisopropylaniline. Interaction of an equimolar ratio of MX2 with aldimine-containing L1 in n-BuOH at 110 °C affords the mononuclear five-coordinate complexes, [(L1)MX2] (M = Fe, X = Cl 1a; M = Co, X = Cl 1b; M = Ni, X = Br 1c; M = Zn, X = Cl 1d), in which the metal centres occupy the terpyridine cavities in L1 with the imino group pendant and exo to the adjacent pyridine nitrogen atom. Similarly, a five-coordinate complex [(L2)ZnCl2] (2d) is accessible from the reaction of ketimine-containing L2 with ZnCl2 with the non-coordinated imine group in 2d, in this case, adopting a pseudo-exo configuration. In contrast, coordination of the imino-nitrogen atom (endo configuration) results on reaction of (DME)NiBr2 (DME = 1,2-dimethoxyethane) with L2 to furnish the six-coordinate complex trans-[(L2)NiBr2] (2c). With [(L2)MCl2] (M = Fe 2a; M = Co 2b) both solution and solid state IR spectroscopy suggest that the imine group prefers to adopt a bound conformation. Quantum mechanical calculations (DFT) have been performed on [(Lx)MX2] (Lx = L1, L2; M = Fe, Co, Ni, Zn; X = Cl, Br) and support the synthetic results with the exo conformations energetically preferred for 1a–1d and 2d, while a distinct energetic preference is observed for the endo conformation in 2c. For 2a and 2b, however, the closeness in energies for endo and exo configurations suggests dynamic behaviour is likely. The iron species 1a and 2a display low activities (on treatment with excess MAO) for ethylene oligomerisation at one bar ethylene pressure affording highly linear -olefins (>98%); the cobalt (1b, 2b) and nickel (1c, 2c) species are inactive. Single crystal X-ray diffraction studies have been performed on 1a, 1b, 1d, 2c and 2d.