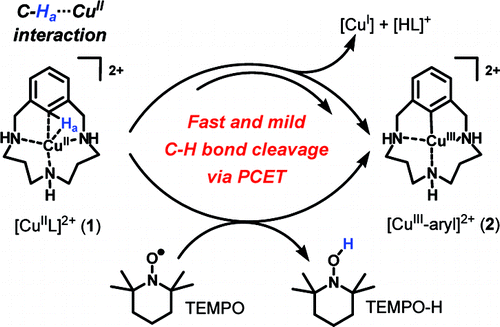
The present study provides mechanistic details of a mild aromatic C-H activation effected by a copper(II) center ligated in a triazamacrocylic ligand, affording equimolar amounts of a CuIII-aryl species and CuI species as reaction products. At low temperatures the CuII complex 1 forms a three-center, three-electron C-H•••CuII interaction, identified by pulse electron paramagnetic resonance spectroscopy and supported by density functional theory calculations. C-H bond cleavage is coupled with copper oxidation, as a CuIII-aryl product 2 is formed. This reaction proceeds to completion at 273 K within minutes through either a copper disproportionation reaction or, alternatively, even faster with 1 equiv of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), quantitatively yielding 2. Kinetic studies of both reactions strongly implicate a rate-limiting proton-coupled electron transfer as the key C-H activation step, a mechanism that does not conform to the C-H activation mechanism in a NiII analogue or to any previously proposed C-H activation mechanisms.