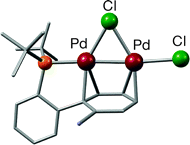
A series of new palladium dinuclear species with general formula [Pd2X(nu-X){nu-PtBu2(Bph-R)}] (X = Cl, Br; Bph = biphenyl; R = H, Me, NMe2) have been prepared. The two palladium centers in these species are bridged by one of the aromatic rings of the biphenyl group present in the corresponding phosphine. The X-ray crystal structure of one of these complexes has been obtained, providing a clear picture of the bonding pattern. The stability of these dimers in solution is shown to be highly dependent on the nature of the phosphine R group and also on the bridging halide. When R = NMe2, the dimers dissociate, yielding the palladium(II) compounds PdX2{PtBu2(BPh-NMe2)} (X = Cl, Br), and the X-ray crystal structure of one of them (X = Br) has shown that the biphenyl group from the phosphine interacts directly with the metal center. This interaction seems to play an important role in stabilizing the otherwise coordinatively unsaturated palladium(II) complex. In contrast, when R = H or Me, the analogous monomeric palladium(II) complexes are unstable and undergo cyclometalation to generate a palladium(II) dinuclear species in which each of the two phosphines cyclometalates with the palladium centers forming a strained four-membered ring. In addition to their unusual structures, these aryl-bridged dimers have also proven to be excellent precatalysts for the amination of aryl chlorides. To rationalize some of the experimental results, a detailed DFT computational study has been carried out and is presented herein.