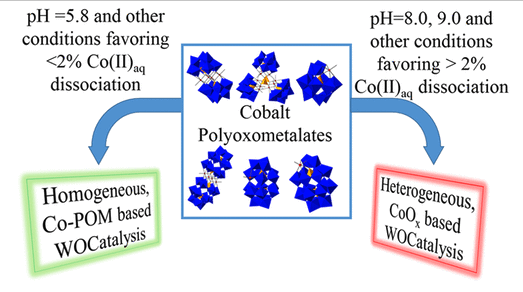
A series of six exemplary cobalt-polyoxometalate (Co-POM) precatalysts have been examined to determine if they are molecular water-oxidation catalysts (WOCatalysts) or if, instead, they actually form heterogeneous, electrode-bound CoOx as the true WOCatalyst under electrochemically driven water-oxidation catalysis (WOCatalysis) conditions. Specifically, WOCatalysis derived from the following six Co-POMs has been examined at pH 5.8, 8.0, and 9.0: [Co4(H2O)2(PW9O34)2]10–(Co4P2W18), [Co9(H2O)6(OH)3(HPO4)2(PW9O34)3]16– (Co9P5W27), [ββ-Co4(H2O)2(P2W15O56)2]16– (Co4P4W30), [Co(H2O)PW11O39]5– (CoPW11), [α1-Co(H2O)P2W17O61]8– (α1-CoP2W17), and [α2-Co(H2O)P2W17O61]8– (α2-CoP2W17). The amount of Co(II)aq in 500 μM solutions of each Co-POM was measured after 3 h of aging as well as from t= 0 for pH = 5.8 and 8.0 by μM sensitive Co(II)aq-induced 31P NMR line broadening and at pH = 9.0 by cathodic stripping. The amount of detectable Co(II)aq after 3 h for the six Co-POMs ranges from ∼0.25 to ∼90% of the total cobalt initially present in the Co-POM. For 12 out of 18 total Co-POM and different pH cases, the amount Co(II)aq detected after 3 h forms heterogeneous CoOxable to account for ≥100% of the observed WOCatalysis activity. However, under 0.1 M NaPi, pH 5.8 conditions for CoPW11 and α1-CoP2W17 where ∼1.5% and 0.25% Co(II)aq is detectable, the measured Co(II)aq cannot account for the observed WOCatalysis. The implication is that these two Co-POMs are primarily molecular, Co-POM-based, WOCatalysts under electrochemically driven, pH 5.8, phosphate-buffer conditions. Even for the single most stable Co-POM, α1-CoP2W17, CoOxis still an estimated ∼76× faster WOCatalyst at pH = 5.8 and an estimated ∼740× faster WOCatalyst at pH = 8.