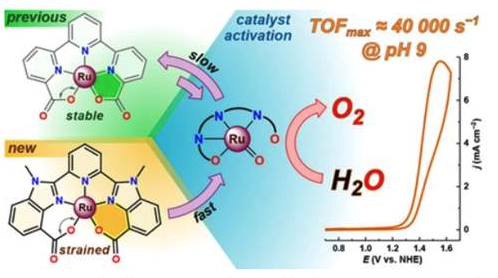
The highly active ruthenium-based water oxidation catalyst [RuX(mcbp)(OHn)(py)2] [mcbp2−=2,6-bis(1-methyl-4-(carboxylate)benzimidazol-2-yl)pyridine; n=2, 1, and 0 for X=II, III, and IV, respectively], can be generated in a mixture of RuIII and RuIV states from either [RuII(mcbp)(py)2] or [RuIII(Hmcbp)(py)2]2+ precursors. The precursor complexes are isolated and characterized by single-crystal X-ray analysis, NMR, UV/Vis, EPR, and FTIR spectroscopy, ESI-HRMS, and elemental analysis, and their redox properties are studied in detail by electrochemical and spectroscopic methods. Unlike the parent catalyst [Ru(tda) (py)2] (tda2−=[2,2′:6′,2′′-terpyridine]-6,6′′-dicarboxylate), for which full transformation into the catalytically active species [RuIV(tda)(O)(py)2] could not be carried out, stoichiometric generation of the catalytically active Ru–aqua complex [RuX(mcbp)(OHn)(py)2] from the RuII precursor was achieved under mild conditions (pH 7.0) and short reaction times. The redox properties of the catalyst were studied and its activity for electrocatalytic water oxidation was evaluated, reaching a maximum turnover frequency (TOFmax) of around 40 000 s−1 at pH 9.0 (from foot-of-the-wave analysis), which is comparable to the activity of the state-of-the-art catalyst [RuIV(tda)(O)(py)2].