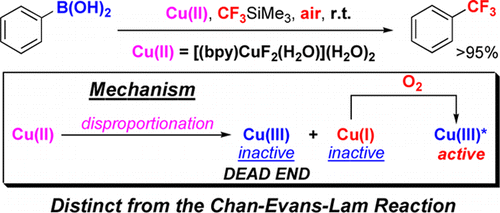
The fluoride [(bpy)CuF2(H2O)]·2H2O (1) reacts with CF3SiMe3 and PhB(OH)2 in DMF at rt to give PhCF3 in >95% yield within 15 min. Although 1 is a Cu(II) complex, this reaction occurs only in air; no Ph-CF3 coupling takes place under anaerobic conditions. A distinct mechanism is operational in this transformation. First, 1 is trifluoromethylated with TMSCF3 to give “[(bpy)Cu(CF3)2]” that spontaneously disproportionates to two Cu(III) ([Cu(CF3)4]− and [(bpy)Cu(CF3)3]) and two Cu(I) ([(bpy)Cu(CF3)] and [Cu(CF3)2]−) complexes. In contrast with the Chan–Evans–Lam reaction, where the Cu(III) products of the Cu(II) disproprotionation effect the coupling, those formed in the 1-TMSCF3 system, [Cu(CF3)4]− and [(bpy)Cu(CF3)3], are stable and unreactive, remaining dead-end spectators throughout the coupling process. Consequently, the trifluoromethylation of PhB(OH)2 with 1-CF3SiMe3 does not and cannot occur in the absence of O2. Only by air oxidation of the Cu(I) disproportionation product, [(bpy)Cu(CF3)] in equilibrium with [Cu(CF3)2]−, is the reactive species generated, serving as a catalyst for the Ph-CF3 bond formation even if 1 is used in stoichiometric quantities.