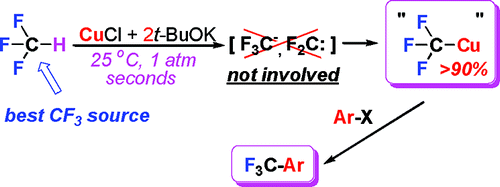
We have found the first reaction of direct cupration of fluoroform, the most attractive CF3 source for the introduction of the trifluoromethyl group into organic molecules. Treatment of CuX (X = Cl, Br, I) with 2 equiv of MOR (M = K, Na) in DMF or NMP produces novel alkoxycuprates that readily react with CF3H at room temperature and atmospheric pressure to give CuCF3 derivatives. The CuCl and t-BuOK (1:2) combination provides best results, furnishing the CuCF3 product within seconds in nearly quantitative yield. As demonstrated, neither CF3– nor CF2 mediate the Cu-CF3 bond formation, which accounts for its remarkably high selectivity. The fluoroform-derived CuCF3 solutions can be efficiently stabilized with TREAT HF to produce CuCF3 reagents that readily trifluoromethylate organic and inorganic electrophiles in the absence of additional ligands such as phenanthroline. A series of novel Cu(I) complexes have been structurally characterized, including K(DMF)[Cu(OBu-t)2] (1), Na(DMF)2[Cu(OBu-t)2] (2), [K8Cu6(OBu-t)12(DMF)8(I)]+ I– (3), and [Cu4(CF3)2(C(OBu-t)2)2(μ3-OBu-t)2] (7).