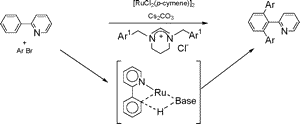
Direct functionalization of sp2 C-H bonds via ortho diarylation of 2-pyridyl benzene with arylbromides was achieved using ruthenium(II) catalysts containing a RuCl2(NHC) unit and generated from [RuCl2(arene)]2 and two types of NHC precursors, pyrimidinium and benzimidazolium salts, in the presence of Cs2CO3. DFT calculations from RuCl2(NHC)(2-pyridylbenzene) show that a proton abstraction mechanism, on cooperative actions of both the coordinated base and the Ru(II) center, is favored via a 13.7 kcal·mol-1 exothermic process affording an orthometalated intermediate with a 2.009 Å Ru-C bond.