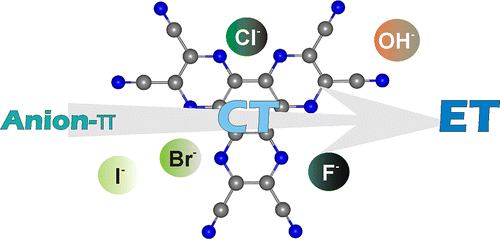
We report experimental evidence indicating that the nature of the interaction established between HAT(CN)6, a well-known strong electron acceptor aromatic compound, with mono- or polyatomic anions switches from the almost exclusive formation of reversible anion-π complexes, featuring a markedly charge transfer (CT) or formal electron-transfer (ET) character, to the quantitative and irreversible net production of the anion radical [HAT(CN)6]•- and the dianion [HAT(CN)6]2- species. The preferred mode of interaction is dictated by the electron donor abilities of the interacting anion. Thus, weaker Lewis basic anions such as Br– or I– are prone to form mainly anion-π complexes. On the contrary, stronger Lewis basic F– or -OH anions display a net ET process. The ET process can be either thermal or photoinduced depending on the HOMO/LUMO energy difference between the electron donor (anion) and the electron acceptor (HAT(CN)6). These ET processes possibly involve the intermediacy of anion-π complexes having strong ET character and producing an ion-pair radical complex. We hypothesize that the irreversible dissociation of the pair of radicals forming the solvent-caged complex is caused by the reduced stability (high reactivity) of the radical resulting from the anion.