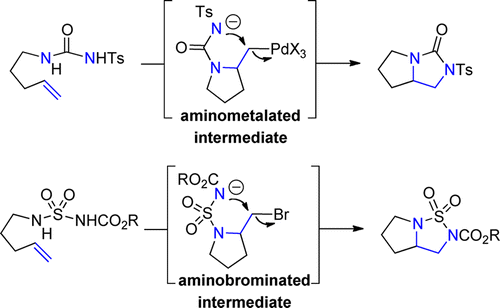
Palladium catalysis has been instrumental in the development of the intramolecular diamination of alkenes. Reagent combinations of a palladium catalyst and iodosobenzene diacetate or copper(II) salts, respectively, represent the broad applicability and mechanistic variation. Recent work has established alternative copper and bromine catalysts. The occupation with this reaction has also contributed to the development of high oxidation state metal catalysis in alkene difunctionalization and significantly broadened the spectrum of Pd-catalyzed C-N bond-forming reactions in general.