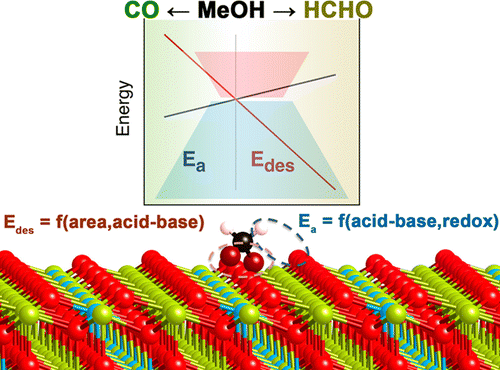
Descriptors are crucial to systematize and optimize the activity, selectivity, and stability of catalysts. Adsorption energies have usually been taken as the main representative parameters that can summarize reaction energies and activation barriers for simple reactions on relatively simple reaction sites. However, more chemically sound terms, which can be directly mapped to experiments, would be more desirable. In addition, larger molecules with more than one potentially active position and complex sites, such as the acid–base pairs present in oxides, are typically beyond the scope provided by common linear-scaling methods. In the present work, we have analyzed the selectivity of the conversion of a polyfunctional molecule on a complex oxide that presents both acid–base and redox chemistry. The conversion of methanol to formaldehyde or CO on isovalently doped ceria(111) has been taken as an example. The selectivity toward CO is triggered by the competition between formaldehyde desorption and C–H cleavage. Our results show that, by introduction of dopant cations, the activation energy of the first H stripping of formaldehyde can be decreased so that its conversion becomes favorable over desorption for Zr- and Hf-doped systems and expanded lattice ceria. More importantly, desorption is controlled by geometric and acid–base factors, whereas C–H cleavage is exclusively electronically governed through acid–base and redox factors. Thus, both geometric and electronic structure parameters are needed to optimize the performance of ceria to attain the desired selectivity. Selectivity is then estimated by a collective descriptor of the surface that incorporates the ensemble size, acid–base, and redox contributions that can be directly compared to experimental values. In addition, this scaling relationship reduces the error associated with more traditional energy-based descriptors. We anticipate that the present scheme can be extended to metal oxides and other polyfunctionalized catalysts.