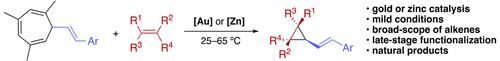Through the design of a second generation of more reactive 7-substituted 1,3,5-cycloheptatrienes, a room-temperature gold(I)-catalyzed retro-Buchner–cyclopropanation sequence and the first zinc(II)-catalyzed version of this process, which uses inexpensive ZnBr2 as catalyst, have been developed. This led to a broad-scope cyclopropanation of both activated and unactivated alkenes, including late-stage derivatization of biologically relevant compounds, and to the total synthesis of (±)-lactobacillic acid.