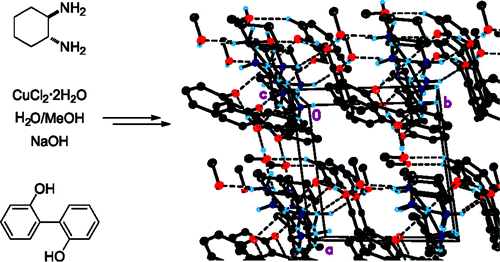
Dynamic atroposelective resolutions in mixed Cu(II) complexes derived from stereolabile biphenyl-2,2′-diol and enantiopure 1,2-diamines have been achieved by crystallization. In these cases, all 2,2′-disubstituted biphenyl fragments in the crystal have the same configuration at the stereogenic axis as a result of the transmission of chirality at the molecular level from the chiral inducer (enantiopure diamine ligand) to the dynamically racemic biaryl units by means of supramolecular forces (metal-ligand and hydrogen bonding interactions). Stereoselective synthetic strategies toward [Cu(2,2′-biphenolate)(1,2-diamine)] complexes have been developed, and these derivatives have been characterized and studied by X-ray diffraction. The formation of hydrogen bonds in an intramolecular (between the biphenyl-2,2′-diol and diamine moieties) and in an intermolecular (between the moieties of the copper complex and cocrystallized methanol) way appears to be essential for the induction of chirality.