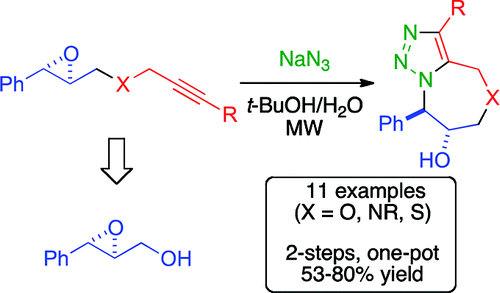
Treatment of alk-2-ynyl derivatives of enantiopure phenylglycidol with NaN3 triggers a cascade reaction consisting of stereospecific and regioselective epoxide ring opening followed by intramolecular azide-alkyne cycloaddition under strictly metal-free conditions. This simple one-pot procedure allows a fast buildup of molecular complexity, generating a wide array of triazolooxazepinols, triazolodiazepinols, and triazolothiazepinols.