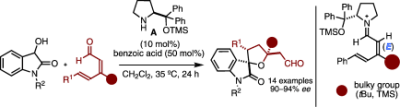
All about topology control: The title reaction yields valuable tetrahydrofuran spirooxindoles (see scheme; TMS=trimethylsilyl), and exemplifies a rare asymmetric 1,6-addition to linear 2,4-dienals proceeding with high δ-site- and stereoselectivity. A steering group at the β-dienal position ensured molecular preorganization of the catalytically active vinylogous iminium ion intermediate for highly predictable reaction outcomes.