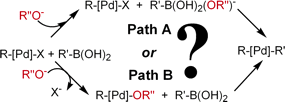The role of the base in the transmetalation step of the Suzuki-Miyaura cross-coupling reaction is analyzed computationally by means of DFT calculations with the Becke3LYP functional. The model system studied consists of Pd(CH=CH2)(PH3)2Br as the starting catalyst complex, CH2=CHB(OH)2 as the organoboronic acid, and OH- as the base. The two main mechanistic proposals, consisting of the base attacking first either the palladium complex or the organoboronic acid, are evaluated through geometry optimization of the corresponding intermediates and transition states. Supplementary calculations are carried out on the uncatalyzed reaction and on a process where the starting complex is Pd(CH=CH2)(PH3)2(OH). These calculations, considered together with available experimental data, strongly suggest that the main mechanism of transmetalation in the catalytic cycle starts with the reaction of the base and the organoboronic acid.