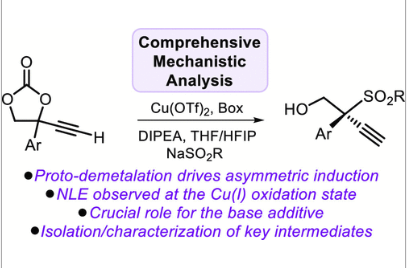
Metal-catalyzed propargylic transformations represent a powerful tool in organic synthesis to achieve new carbon–carbon and carbon–heteroatom bonds. However, detailed knowledge about the mechanistic intricacies related to the asymmetric formation of propargylic products featuring challenging heteroatom-substituted tertiary stereocenters is scarce and therefore provides an inspiring challenge. Here, we present a meticulous mechanistic analysis of a propargylic sulfonylation reaction promoted by a chiral Cu catalyst through a combination of experimental techniques and computational studies. Surprisingly, the enantio-discriminating step is not the coupling between the nucleophile and the propargylic precursor but rather the following proto-demetalation step, a scenario further validated by computing enantio-induction levels under other previously reported experimental conditions. A full mechanistic scenario for this propargylic substitution reaction is provided, including a catalyst pre-activation stage, a productive catalytic cycle, and an unanticipated non-linear effect at the Cu(I) oxidation level.