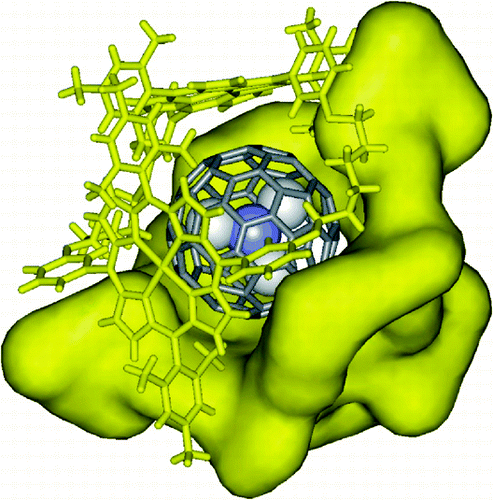
The authors report the synthesis of two cyclic β-pyrrole unsubstituted meso-tetraphenyl bisporphyrins in which the porphyrin units are connected by two 2,3-hexadiynyl-1,6-dioxo or two hexyl-1,6-dioxo spacers, respectively. Both cyclic porphyrin dimers exist in solution as mixtures of two conformational isomers. In the solid state, the receptor with diynyl spacers forms a 1:1 complex with the icosahedral (Ih) isomer of the trimetallic nitride endohedral fullerene Sc3N @ C80. In this complex the receptor adopts a scoop-shaped conformation having a dihedral angle of 87.25° between the two porphyrin planes. The hexyl spaced analog, however, adopts a similar conformation upon encapsulation of one molecule of Sc3N @ C80 in a self-assembled dimeric capsule. The capsular complexes pack in columns and render the fullerene units completely isolated. In toluene solution, 1H NMR experiments indicate that the endohedral fullerene Sc3N @ C80 is exclusively bound by the expanded isomer of both dimers. UV-visible and fluorescence titration experiments confirmed the existence of strong π-π interactions between the fullerene Sc3N @ C80 and the flexible bisporphyrin dimer with hexyl spacers. At micromolar concentration, the flexible receptor forms only a 1:1 complex with the endohedral fullerene with stability const. value of Ka = 2.6 ± 0.3 × 105 M-1.