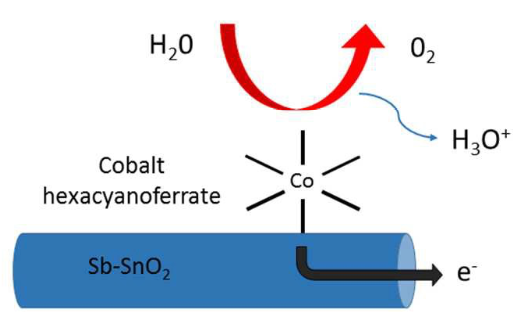
This study investigates the activity and stability of a prussian blue analogue (PBA) as an inexpensive anode catalyst for Polymer Electrolyte Membrane Water Electrolysis (PEMWE). While some PBAs have recently been reported to catalyze the oxygen evolution reaction (OER) in acidic electrolytes, the present study focuses on their integration in a PEMWE device. Cobalt hexacyanoferrate nanoparticles were interfaced with an electrically conductive support that withstands the PEMWE anodic conditions, namely Sb-doped SnO2. The OER activity of the composite materials was first verified in liquid electrolytes and then in PEMWE. A promising current density of 50-100 mA cm-2 was reached at 2 V cell voltage. The PBA/Sb-SnO2 anode was stable up to 1.9 V, but showed more and more instability at higher potentials. Increasing leaching rates of Sn and Sb observed above 1.9 V suggest that the material instability above 1.9 V can mainly be assigned to Sb-doped SnO2 conductive support. These results are overall promising for the use of PBAs as catalytic sites at the anode of PEMWE. The study also identifies the need for more active PBAs in order to reach a higher current density at a cell voltage of 1.6-1.9 V, a potential range necessary for an acceptable energy efficiency of the PEMWE.