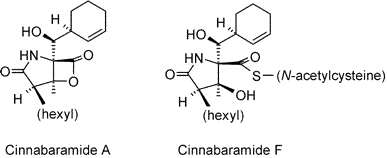
The cinnabaramides A-G (1–7) were isolated from a terrestrial strain of Streptomyces as potent and selective inhibitors of the human 20S proteasome. Their chemical and biological properties resemble those of salinosporamide A, a recently identified lead compound from an obligate marine actinomycete, which is currently under development as an anticancer agent. Cinnabaramides F and G (6, 7) combine essential structural features of salinosporamide A and lactacystin and show about equal potency in vitro, with IC50 values in the 1 nM range. The properties and phylogenetic position of the producer organism, the production and isolation of compounds 1–7, their structure elucidation by MS and NMR, and their biological activities are reported. Additionally, an X-ray crystal structure was obtained from cinnabaramide A (1).