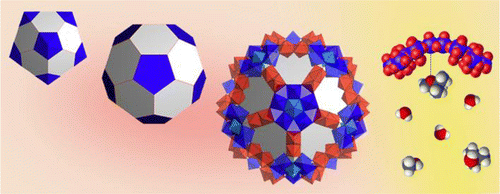
The 30 cationic {MoV2O4(acetate)}+ units linking 12 negatively charged pentagonal “ligands,” {(MoVI)MoVI5O21(H2O)6}6- of the porous metal-oxide capsule, [{MoVI6O21(H2O)6}12{MoV2O4(acetate)}30]42- provide active sites for catalytic transformations of organic “guests”. This is demonstrated using a well-behaved model reaction, the fully reversible cleavage and formation of methyl tert-butyl ether (MTBE) under mild conditions in water. Five independent lines of evidence demonstrate that reactions of the MTBE guests occur in the ca. 6 × 103 Å3 interior of the spherical capsule. The Mo atoms of the {MoV2O4(acetate)}+ linkers-spanning an ca. 3-nm truncated icosahedron-are sterically accessible to substrate, and controlled removal of their internally bound acetate ligands generates catalytically active {MoV2O4(H2O)2}2+ units with labile water ligands, and Lewis- and Brønsted-acid properties. The activity of these units is demonstrating by kinetic data that reveal a first-order dependence of MTBE cleavage rates on the number of acetate-free {MoV2O4(H2O)2}2+ linkers. DFT calculations point to a pathway involving both Mo(V) centers, and the intermediacy of isobutene in both forward and reverse reactions. A plausible catalytic cycle-satisfying microscopic reversibility-is supported by activation parameters for MTBE cleavage, deuterium and oxygen-18 labeling studies, and by reactions of deliberately added isobutene and of a water-soluble isobutene analog. More generally, pore-restricted encapsulation, ligand-regulated access to multiple structurally integral metal-centers, and options for modifying the microenvironment within this new type of nanoreactor, suggest numerous additional transformations of organic substrates by this and related molybdenum-oxide based capsules.