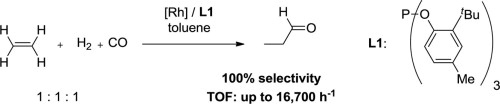
A wide range of monophosphite ligands was investigated in rhodium-catalyzed ethene hydroformylation. A stoichiometric gas mixture CO/H2/ethene 1:1:1 was used, the reaction being thus 100% atom economic. The reaction was found to be very selective and only propanal was formed under the reaction conditions studied. The most efficient catalytic system was L1-modified rhodium, and reaction parameters were optimized for this ligand. Under optimized catalytic conditions, reaction rates 10-15 times higher than those of the triphenylphosphine-modified system were obtained, demonstrating the high suitability of π-accepting ligands for this reaction. Stability tests, resistance toward water and acids in particular, showed the good stability of the selected phosphite L1. Notably, L1 was more stable than cyclic phosphites L6 and L13.