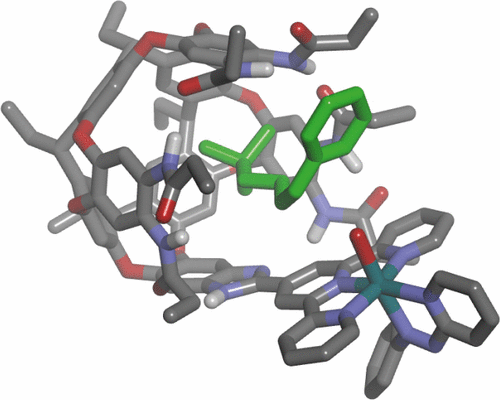
We report the design, synthesis and characterization of a new RuII metallocavitand that is catalytically active in alkene epoxidation reactions. The elaboration of the resorcin[4]arene’s aromatic cavity produced a self-folding, deep hexaamide cavitand featuring a single diverging terpyridine (tpy) group installed at its upper rim. The construction of the metallocavitand involved the initial chelation of a RuIII chloride complex by the tpy ligand followed by the incorporation of 2-(phenylazo)pyridine (azpy) as an ancillary ligand. The resulting RuII chloro complex was converted into the catalytically active aqua counterpart by a ligand exchange process.