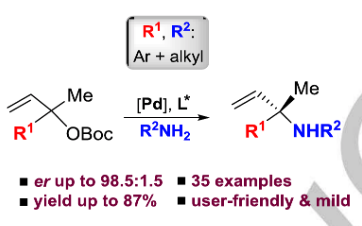
The first asymmetric synthesis of important alfa,alfa-disubstituted N-alkyl allyl amine scaffolds via allylic substitution is reported. This approach is based on palladium catalysis and features ample scope in both allylic precursor and amine reagent, and high asymmetric induction with the enantiomeric ratio (er) up to 98.5:1.5. The use of less reactive anilines is also feasible providing enantioenriched alfa,alfa-disubstituted N-aryl allylic amines.