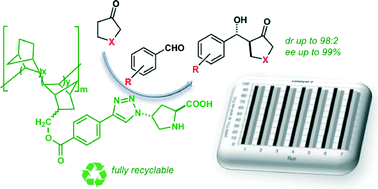
In an effort to identify novel polymer architectures suitable for a covalent support for catalysts, L-proline derivatives have been immobilized onto rationally designed vinyl addition polynorbornene (VA-PNB) resins through copper-catalyzed azide–alkyne cycloaddition (CuAAC) reactions. These fully saturated resins have been found to be optimal catalyst supports, the resulting proline-functionalized resins behave as very active, easily recoverable and highly reusable organocatalysts for the asymmetric direct aldol reaction of benzaldehydes with ketones in aqueous media. The results show that, the combination of modular VA-PNB resins with proline derivatives and triazole linkers represents a promising strategy for the immobilization of organocatalytic species.