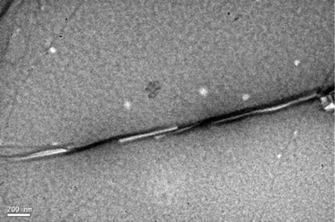
The chiral bicyclic diguanidinium chloride 1 forms gels in aromatic apolar solvents. The gels were characterized at different levels of organization, from the macroscopic to the molecular level by using microscopy, spectroscopy, and powder X-ray diffraction. The dependency on chirality has been highlighted by circular dichroism and electron microscopy. Furthermore, the gel has been shown to be effectively responsive to anionic stimuli, thus allowing the reversible control of the organic-phase gelation in contact with different salted aqueous solutions.