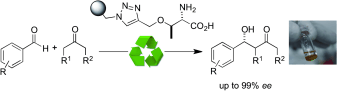
A series of primary amino acids covalently supported onto polystyrene through alkyne–azide cycloaddition reactions has been synthesized and evaluated as catalysts in asymmetric aldol reactions. A polymer-supported threonine behaves as an easily recyclable, highly reactive and stereoselective (up to 99% ee) catalyst in the aldol reaction of both cyclic and acyclic ketone donors with aromatic aldehydes in aqueous environments. While cyclic ketones react with anti diastereoselectivity, syn adducts are predominantly obtained with acyclic substrates. The heterogenized threonine catalyst has been used for the sequential synthesis of a small library of enantiopure aldol products.