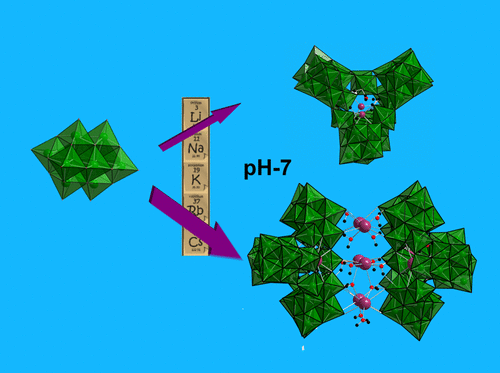
Counterions are deemed “spectators” in aqueous solutions of cationic or anionic molecular metal-oxo clusters. While pH and concentration drive aqueous metal speciation as a first approximation, the important effect of counterions is usually overlooked and never considered in standard Pourbaix databases. Alkali counterions for polyoxometalate (POM) clusters control solubility with distinct periodic trends, but evidence for alkali control over speciation is ambiguous. Here we show that a simple Nb-POM, [Nb10O28]6– ({Nb10}), converts to oligomers of (HxNb24O72)(24–x)– ({Nb24}) upon adding only alkali chloride salts, even in buffered neutral solutions. Raman and X-ray scattering reveal that the rate of {Nb10} to {Nb24} conversion increases with alkali cation radius and cation concentration. Cation-bridged oligomers of {Nb24}y (y = 2,4) are defined by comparing experimental to computed small-angle X-ray scattering spectra. Computational studies and mass spectrometry indicate that the alkalis open the compact {Nb10} cluster in conjunction with protonation of a heptamer {Nb7} intermediate, in which alkali-{Nb10} association at key locations on the cluster initiates the reaction. Computation also explains the alkali periodic trend for {Nb10} to {Nb24} conversion; larger alkalis more effectively destabilize {Nb10}. This periodic trend asserts the hypothesis that Nb-cluster speciation near neutral pH is driven by the alkali cations in the absence of added base or acid. The extremely high solubility of these 3.5 nm polyoxoanion assemblies—2 M Nb at near neutral pH—is both surprising and exploitable for aqueous synthesis of niobate thin films or nanomaterials used in energy and microelectronics applications.