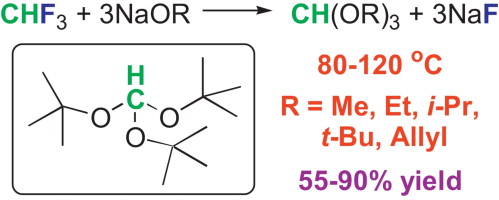
Fluoroform (CHF3) reacts with alkali metal alkoxides MOR (M = Na, K) in the corresponding alcohols ROH (R = Me, Et, i-Pr, t-Bu, and Allyl) at 80–120 °C to give orthoformate esters HC(OR)3 in 55–90% yield. Particularly notable is the formation of HC(OBu-t)3 in 75–80% yield (62% isolated yield; X-ray), an exotic organic compound that has been previously synthesized only once (3% yield). Solutions of NaOH in ROH (R = Me, Et, i-Pr and t-Bu) react with CHF3 to give NaF, HCOONa, and orthoformates HC(OR)3. Hydrolysis of fluoroform with MOH (M = Na, K) at 140 °C produces largely MF and HCOOM along with small quantities of CO.