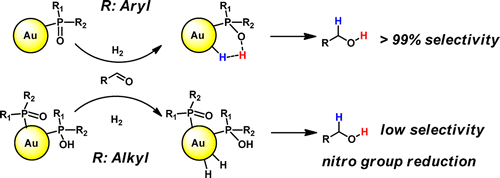
Air-stable and homogeneous gold nanoparticles (AuNPs, 1a–5a) ligated by various secondary phosphine oxides (SPOs), [R1R2P(O)H] (R1 = Naph, R2 = tBu, L1; R1 = R2 = Ph, L2; R1 = Ph, R2 = Naph, L3; R1 = R2 = Et, L4; R1 = R2 = Cy, L5; R1 = R2 = tBu, L6), with different electronic and steric properties were synthesized via NaBH4 reduction of the corresponding Au(I)–SPO complex. These easily accessible ligands allow the formation of well dispersed and small nanoparticles (size 1.2–2.2 nm), which were characterized by the use of a wide variety of techniques, such as transmission electron microscopy, thermogravimetric analysis, UV–vis, energy-dispersive X-ray, X-ray photoelectron spectroscopy (XPS), attenuated total reflectance Fourier transform infrared spectroscopy (ATR FT-IR), and cross polarization magic angle spinning (CP MAS) NMR spectroscopy. A pronounced ligand effect was found, and CP MAS NMR experiments enabled us to probe important differences in the polarity of the P–O bond of the SPOs coordinated to the nanoparticle surface depending on the type of substituents in the ligand. AuNPs containing aryl SPOs carry only SPO anions and are highly selective for aldehyde hydrogenation. AuNPs of similar size made with alkyl SPOs contain also SPOH, hydrogen bonded to SPO anions. As a consequence they contain less Au(I) and more Au(0), as is also evidenced by XPS. They are less selective and active in aldehyde hydrogenation and now show the typical activity of Au(0)NPs in nitro group hydrogenation.