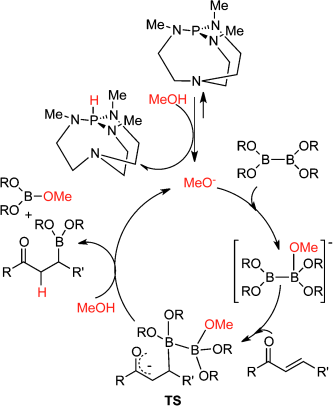
Bases play an important role in organocatalytic boron conjugate addition reactions. The sole use of MeOH and a base can efficiently transform acyclic and cyclic activated olefins into the corresponding β-borated products in the presence of diboron reagents. Inorganic and organic bases deprotonate MeOH in the presence of diboron reagents. It is concluded, on the basis of theoretical calculations, NMR spectroscopic data, and ESI-MS experiments, that the methoxide anion forms a Lewis acid-base adduct with the diboron reagent. The sp2 B atom of the methoxide-diboron adduct gains a strongly nucleophilic character, and attacks the electron-deficient olefin. The methanol protonates the intermediate, generating the product and another methoxide anion. This appears to be the simplest method to activate diboron reagents and make them suitable for incorporation into target organic molecules.