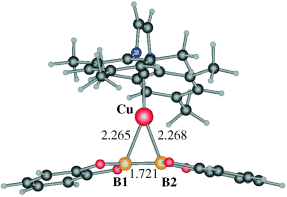
The complexes [Cu(NHC)(NCMe)]BF4 (NHC=N-heterocyclic ligand), with bis(catecholato)diboron (B2(cat)2) as the boron source, efficiently catalyze the diboration of styrene with very high degrees of conversion. With the appropriate NHC ligand, the reaction proceeds quantitatively toward the diborated derivative PhCH(Bcat)CH2(Bcat). The [styrene]/[B2(cat)2] ratio also has a strong effect on the selectivity: the use of an excess of styrene allows modification of the selectivity toward the formation solely of the monoborated derivative, PhCH2CH2(Bcat). DFT calculations suggest that no oxidative addition processes take place at copper, but that intermediates containing coordinated -bonds are involved in the catalytic cycle.