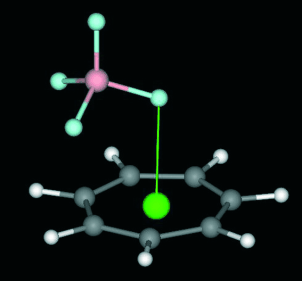
Several complexes of tropylium (1) with anions are optimized at the RI-MP2(full)/6-31++G** level of theory. This binding unit can interact very favorably with anions, and it combines the strength of the electrostatic interaction with the directionality of the anion-pi interaction. The complexes of 1 with anions are characterized by means of the Bader theory of atoms-in-molecules, and the physical nature of the interaction has been analyzed by means of the molecular interaction potential with polarization tool. Experimental evidence of anion-pi interactions involving seven-membered rings has been found in the solid state.